
Uranium (pronounced /jʊˈreɪniəm/) is a silvery-white metallic chemical element in the actinide series of the periodic table that has the symbol U and atomic number 92. Besides its 92 protons, a uranium nucleus can have between 141 and 146 neutrons, with 146 (U-238) and 143 (U-235) in its most common isotopes. The number of electrons in a uranium atom is 92, 6 of them valence electrons. Uranium has the highest atomic weight of the naturally occurring elements. Uranium is approximately 70% denser than lead, but not as dense as gold or tungsten. It is weakly radioactive. It occurs naturally in low concentrations (a few parts per million) in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite (see uranium mining).
In nature, uranium atoms exist as uranium-238 (99.284%), uranium-235 (0.711%),and a very small amount of uranium-234 (0.0058%). Uranium decays slowly by emitting an alpha particle. The half-life of uranium-238 is about 4.47 billion years and that of uranium-235 is 704 million years, making them useful in dating the age of the Earth (see uranium-thorium dating, uranium-lead dating and uranium-uranium dating).
Many contemporary uses of uranium exploit its unique nuclear properties. Uranium-235 has the distinction of being the only naturally occurring fissile isotope. Uranium-238 is both fissionable by fast neutrons, and fertile (capable of being transmuted to fissile plutonium-239 in a nuclear reactor). An artificial fissile isotope, uranium-233, can be produced from natural thorium and is also important in nuclear technology. While uranium-238 has a small probability to fission spontaneously or when bombarded with fast neutrons, the much higher probability of uranium-235 and to a lesser degree uranium-233 to fission when bombarded with slow neutrons generates the heat in nuclear reactors used as a source of power, and provides the fissile material for nuclear weapons. Both uses rely on the ability of uranium to produce a sustained nuclear chain reaction. Depleted uranium (uranium-238) is used in kinetic energy penetrators and armor plating.
Uranium is used as a colorant in uranium glass, producing orange-red to lemon yellow hues. It was also used for tinting and shading in early photography. The 1789 discovery of uranium in the mineral pitchblende is credited to Martin Heinrich Klaproth, who named the new element after the planet Uranus. Eugène-Melchior Péligot was the first person to isolate the metal, and its radioactive properties were uncovered in 1896 by Antoine Becquerel. Research by Enrico Fermi and others starting in 1934 led to its use as a fuel in the nuclear power industry and in Little Boy, the first nuclear weapon used in war. An ensuing arms race during the Cold War between the United States and the Soviet Union produced tens of thousands of nuclear weapons that used enriched uranium and uranium-derived plutonium. The security of those weapons and their fissile material following the breakup of the Soviet Union in 1991 is an ongoing concern for public health and safety.
From http://en.wikipedia.org/
-
Ear Force X41 (XBOX LIVE Chat + Wireless Digital RF Game Audio with Dolby Headphone 7.1 Surround Sound) - Ear Force X41 (XBOX LIVE Chat + Wireless Digital RF Game Audio with Dolby Headphone 7.1 Surround Sound) by Turtle Beach [image: Ear Force X41 (XBOX LIVE C...
Sunday, August 2, 2009
Uranium
Labels: Materials mined
Posted by my blog at 5:45 AM 0 comments
Tin

Tin is a chemical element with the symbol Sn (Latin: Stannum) and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows chemical similarity to both neighboring group 14 elements, germanium and lead, like the two possible oxidation states +2 and +4. Tin is the 49th most abundant element and has, with 10 isotopes, the largest number of stable isotopes in the periodic table. Tin is obtained chiefly from the mineral cassiterite, where it occurs as tin dioxide, SnO2.
This silvery, malleable poor metal is not easily oxidized in air, and is used to coat other metals to prevent corrosion. The first alloy used in large scale since 3000 BC was bronze, an alloy of tin and copper. After 600 BC pure metallic tin was produced. Pewter, which is an alloy of 85 % to 90 % tin with the remainder commonly consisting of copper, antimony and lead, was used for flatware from the Bronze Age until the 20th century. In modern times tin is used in many alloys, most notably tin/lead soft solders, typically containing 60% or more of tin. Another large application for tin is corrosion-resistant tin plating of steel. Due to its low toxicity, tin-plated metal is also used for food packaging, giving the name to tin cans, which are made mostly out of aluminium or tin-plated steel.
Characteristics
Physical and allotropes
Tin is a malleable, ductile, and highly crystalline silvery-white metal. It is malleable at ordinary temperatures but is brittle when it is cooled, due to the properties of its two major allotropes, α- and β-tin. When a bar of tin is bent, a crackling sound known as the tin cry can be heard due to the twinning of the crystals. The two allotropes that are encountered at normal pressure and temperature, α-tin and β-tin, are more commonly known as gray tin, and respectively white tin. Two more allotropes, γ and σ, exist at temperatures above 161 °C and pressures above several GPa. White tin, or the β-form, is metallic, and is the stable one at room conditions or at higher temperatures. Below 13.2 °C, tin exists in the gray α-form, which has a diamond cubic crystal structure, similar to diamond, silicon or germanium. Gray tin has no metallic properties at all, is a dull-gray powdery material, and has few uses, other than a few specialized semiconductor applications.
Although the α-β transformation temperature is nominally 13.2 °C, impurities (e.g. Al, Zn, etc.) lower the transition temperature well below 0 °C, and upon addition of Sb or Bi the transformation may not occur at all. This conversion is known as tin disease or tin pest. Tin pest was a particular problem in northern Europe in the 18th century as organ pipes made of tin alloy would sometimes be affected during long cold winters. Some sources also say that during Napoleon's Russian campaign of 1812, the temperatures became so cold that the tin buttons on the soldiers' uniforms disintegrated, contributing to the defeat of the Grande Armée. The veracity of this story is debatable, because the transformation to gray tin often takes a reasonably long time.
Commercial grades of tin (99.8%) resist transformation because of the inhibiting effect of the small amounts of bismuth, antimony, lead, and silver present as impurities. Alloying elements such as copper, antimony, bismuth, cadmium, and silver increase its hardness. Tin tends rather easily to form hard, brittle intermetallic phases, which are often undesirable. It does not form wide solid solution ranges in other metals in general, and there are few elements that have appreciable solid solubility in tin. Simple eutectic systems, however, occur with bismuth, gallium, lead, thallium, and zinc.
Chemistry and compounds
Tin is classified as a semimetal, as its chemical properties fall between those of metals and non-metals, just as the semiconductors silicon and germanium do. It resists corrosion from distilled, sea and soft tap water, but can be attacked by strong acids, alkalis, and acid salts. Tin can be highly polished and is used as a protective coat for other metals in order to prevent corrosion or other chemical action. Tin acts as a catalyst when oxygen is in solution and helps accelerate chemical attack.
Tin forms the dioxide SnO2 (cassiterite) when it is heated in the presence of air. SnO2, in turn, is feebly acidic and forms stannate (SnO32−) salts with basic oxides. There are also stannates with the structure [Sn(OH)6]2−, like K2[Sn(OH)6], although the free stannic acid H2[Sn(OH)6] is unknown.
Tin combines directly with chlorine forming tin(IV) chloride, while reacting tin with hydrochloric acid in water gives tin(II) chloride and hydrogen. Several other compounds of tin exist in the +2 and +4 oxidation states, such as tin(II) sulfide and tin(IV) sulfide (Mosaic gold). There is only one stable hydride, however: stannane (SnH4), where tin is in the +4 oxidation state.
The most important salt is stannous chloride, which has found use as a reducing agent and as a mordant in the calico printing process. Electrically conductive coatings are produced when tin salts are sprayed onto glass. These coatings have been used in panel lighting and in the production of frost-free windshields.
Tin is added to some dental care products as stannous fluoride (SnF2). Stannous fluoride can be mixed with calcium abrasives while the more common sodium fluoride gradually becomes biologically inactive combined with calcium. It has also been shown to be more effective than sodium fluoride in controlling gingivitis.
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin compounds usually have high toxicity and have been used as biocides, but their use is slowly being phased out. The first organotin compound was diethyltin diiodide (Sn(C2H5)2I2), discovered by Edward Frankland in 1849. Organotin compounds differ from their lighter analogues of germanium and silicon in that there is a greater occurrence of the +2 oxidation state due to the "inert pair effect"; it also has a greater range of coordination numbers, and the common presence of halide bridges between polynuclear compounds. Most organotin compounds are colorless liquids or solids that are usually stable to air and water. The tetraalkyl stannates (R4Sn) always have a tetrahedral geometry at the tin atom. The halide derivatives R3SnX often form chained structures with Sn-X-Sn bridges. Alkyltin compounds are usually prepared via Grignard reagent reactions such as in:
SnCl4 + 4 RMgBr → R4Sn + 4 MgBrCl.
Isotopes
Tin is the element with the greatest number of stable isotopes, ten; these include all those with atomic masses between 112 and 124, with the exception of 113, 121 and 123. Of these, the most abundant ones are 120Sn (at almost a third of all tin), 118Sn, and 116Sn, while the least abundant one is 115Sn. The isotopes possessing even atomic numbers have no nuclear spin while the odd ones have a spin of +1/2. Tin, with its three common isotopes 115Sn, 117Sn and 119Sn, is among the easiest elements to detect and analyze by NMR spectroscopy, and its chemical shifts are referenced against SnMe4.
This large number of stable isotopes is thought to be a direct result of tin possessing an atomic number of 50, which is a "magic number" in nuclear physics. There are 28 additional unstable isotopes that are known, encompassing all the remaining ones with atomic masses between 99 and 137. Aside from 126Sn, which has a half-life of 230,000 years, all the radioactive isotopes have a half-life of less than a year. The radioactive 100Sn is one of the few nuclides possessing a "doubly magic" nucleus and was discovered relatively recently, in 1994. Another 30 metastable isomers have been characterized for isotopes between 111 and 131, the most stable of which being 121mSn, with a half-life of 43.9 years.
Etymology
The Latin name Stannum is connected to "stagnum" and "stag" (Indo-European) for dripping because tin melts easily. The former "stagnum" was the word for a stale pool or puddle, with a cognate in the English word "stagnant." The English word "tin" has cognates in many Germanic and Celtic languages. The American Heritage Dictionary speculates that the word was borrowed from a pre-Indo-European language. The later name "stannum" and its Romance derivatives come from the lead-silver alloy of the same name for the finding of the silver in ores. The word definitely assumed its present meaning in the 4th century (H. Kopp).
According to Meyers Konversationslexikon Stannum is derived from Cornish stean (present orthography sten), and is proof that Cornwall in the first centuries AD was the main source of tin. Other sources, however, see the Cornish stean merely as a back-derivation from the Latin stannum. The Latin Stannum became the source for most European words. According to SMI the English word for the metal is named after an Etruscan god, Tinia. (variants include Old English: tin, Old Latin: plumbum candidum ("white lead"), Old German: tsin, Late Latin: stannum)
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 5:43 AM 0 comments
Friday, July 31, 2009
Sodium chloride

Sodium chloride, also known as common salt, table salt, or halite, is an ionic compound with the formula NaCl. Sodium chloride is the salt most responsible for the salinity of the ocean and of the extracellular fluid of many multicellular organisms. As the major ingredient in edible salt, it is commonly used as a condiment and food preservative.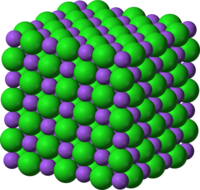
Production and use
Salt is currently mass-produced by evaporation of seawater or brine from other sources, such as brine wells and salt lakes, and by mining rock salt, called halite. In 2002, world production was estimated at 210 million metric tonnes, the top five producers (in million tonnes) being the United States (40.3), China (32.9), Germany (17.7), India (14.5) and Canada (12.3).
As well as the familiar uses of salt in cooking, salt is used in many applications, from manufacturing pulp and paper, to setting dyes in textiles and fabric, to producing soaps, detergents, and other bath products. It is the major source of industrial chlorine and sodium hydroxide, and used in almost every industry.
Sodium chloride is sometimes used as a cheap and safe desiccant because it appears to have hygroscopic properties, making salting an effective method of food preservation historically. Even though more effective desiccants are available, few are safe for humans to ingest.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 6:31 PM 0 comments
Slate

Slate is a fine-grained, foliated, homogeneous metamorphic rock derived from an original shale-type sedimentary rock composed of clay or volcanic ash through low grade regional metamorphism. The result is a foliated rock in which the foliation may not correspond to the original sedimentary layering. Slate is frequently grey in colour especially when seen en masse covering roofs. However, slate occurs in a variety of colours even from a single locality. For example slate from North Wales can be found in many shades of grey from pale to dark and may also be purple, green or cyan. Slate is not to be confused with shale, from which it may be formed, or schist.
Chemical composition
Slate is mainly composed of quartz and muscovite or illite, often along with biotite, chlorite, hematite, and pyrite and, less frequently, apatite, graphite, kaolin, magnetite, tourmaline, or zircon as well as feldspar. Occasionally, as in the purple slates of North Wales, ferrous reduction spheres form around iron nuclei, leaving a light green spotted texture. These spheres are sometimes deformed by a subsequent applied stress field to ovoids, which appear as ellipses when viewed on a cleavage plane of the specimen.
Uses
Slate in buildings
Slate can be made into roofing slates, also called roofing shingles, installed by a slater. Slate has two lines of breakability: cleavage and grain. This makes it possible to split slate into thin sheets. When broken, slate produces a natural appearance while remaining relatively flat and can be easily stacked. Silicone glue adheres to slate.
Slate tiles are often used for interior and exterior flooring, stairs, walkways, and wall cladding. Tiles are installed and set on mortar and grouted along the edges. Chemical sealants are often used on tiles to improve durability and appearance, increase stain resistance, reduce efflorescence, and increase or reduce surface smoothness. Tiles are often sold gauged, meaning that the back surface is ground for ease of installation. Slate flooring can however be slippery when used in external locations subject to rain. Slate tiles were used in 19th century UK building construction (apart from roofs) and in slate quarrying areas such as Bethesda there are still many buildings wholly constructed of slate. Slates can also be set into walls to provide a rudimentary damp-proof membrane. Small offcuts are used as shims to level floor joists. In areas where slate is plentiful it is also used in pieces of various sizes for building walls and hedges, sometimes combined with other kinds of stone.
Other uses
Because it is a good electrical insulator and fireproof, it was used to construct early 20th century electric switchboards and relay controls for large electric motors. Fine slate can also be used as a whetstone to hone knives.
Due to its thermal stability and chemical inertness, slate has been used for laboratory bench tops and for billiard table tops. In 18th and 19th century schools, slate was extensively used for blackboards and individual writing slates for which slate or chalk pencils were used. They were largely used in the 20th century, though writing slates were largely replaced by lined paper and notebooks, and slates still continue wide usage, though they are sometimes replaced with whiteboards.
Where slate of fine quality is available it is used for gravestones and commemorative tablets and by artists in various genres. British sculptor Stephen Kettle is notable for his use of slate to create statues housed in the Science Museum in London.
Slate is often used as a decor in freshwater aquariums. Slate will not alter the chemistry of water (except in the slate containing feldspar which may leach silicates into the water resulting in excess diatom growth in marine aquaria). Traditional Japanese Go equipment uses slate for the black pieces.
Slate extraction
In Eurasia
Slate-producing regions in Europe include Wales (see slate industry in Wales), Cornwall (famously the village of Delabole), and Cumbria (see Burlington Slate Quarries, Honister Slate Mine and Skiddaw Slate) in the United Kingdom; parts of France (Anjou, Ardennes, Bretagne, Savoie); Belgium (Ardenne); Liguria in northern Italy especially between the town of Lavagna (which means chalkboard in Italian) and Fontanabuona valley; Portugal especially around Valongo in the north of the country; Germany's (Moselle River-region, Hunsrück, Eifel, Westerwald, Thuringia and north-Bavaria); Alta, Norway (actually schist not a true slate) and Galicia. Some of the slate from Wales and Cumbria is colored slate (non-blue): (purple and formerly green in Wales) and (green in Cumbria). China has vast slate deposits; in recent years its export of finished and unfinished slate has increased: it has slate in various colors.
In the Americas
Slate is abundant in Brazil (the second biggest producer of slate) around Papagaio in Minas Gerais (responsible for 95% of the extraction of slate in Brazil), the east coast of Newfoundland, the Slate Belt of Eastern Pennsylvania, and the Slate Valley of Vermont and New York. The area around Granville, NY, is one place where colored slate (non-blue) is mined.
There was also a major slating operation in Monson, Maine during the late 19th and early 20th centuries. The slate found in Monson is usually a dark purple to blackish color, and many local structures are still roofed with slate tiles. The roof of St. Patrick's Cathedral was made of roofing slate from Monson, as was the headstone of John F. Kennedy.
Slate is also found in the Arctic and was used by the Inuit to make the blades for ulus.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 6:27 PM 0 comments
Wednesday, July 29, 2009
Sand mining
Sand mining is a practice that is becoming an ecological problem as the demand for sand increases in industry and construction. Sand is mined from beaches and inland dunes and dredged from ocean beds and river beds. It is often used in manufacturing as an abrasive, for example, and it is used to make concrete. As communities grow, construction requires less wood and more concrete, leading to a demand for low-cost sand. Sand is also used to replace eroded coastline.
A related process is the mining of mineral sands, such as mineral deposits, grain, wheat, diamond which contain industrial useful minerals, mainly gold and silver. These minerals typically occur combined with ordinary sand. The sand is dug up, the valuable minerals are separated in water by using their different density, and the remaining ordinary sand is re-deposited.
Sand mining is a direct and obvious cause of erosion, and also impacts the local wildlife. For example, sea turtles depend on sandy beaches for their nesting, and sand mining has led to the near extinction of ghariyals (a species of crocodiles) in India. Disturbance of underwater and coastal sand causes turbidity in the water, which is harmful for such organisms as corals that need sunlight. It also destroys fisheries, causing problems for people who rely on fishing for their livelihoods.
Removal of physical coastal barriers such as dunes leads to flooding of beachside communities, and the destruction of picturesque beaches causes tourism to dissipate. Sand mining is regulated by law in many places, but is still often done illegally.
Sand mining by country
Australia
New South Wales
In the 1930s mining operations began on the Kurnell Peninsula (Captain Cook's landing place in Australia) to supply the expanding Sydney building market. It continued until 1990 with an estimate of over 70 million tonnes of sand having been removed. The sand has been valued for many decades by the building industry, mainly because of its high crushed shell content and lack of organic matter, it has provided a cheap source of sand for most of Sydney since sand mining operations began. The site has now been reduced to a few remnant dunes and deep water-filled pits which are now being filled with demolition waste from Sydney's building sites. Removal of the sand has significantly weakened the peninsula's capacity to resist storms. Ocean waves pounding against the reduced Kurnell dune system have threatened to break through to Botany Bay, especially during the storms of May and June back in 1974 and of August 1998.
Queensland
A large and long running sandmine in Queensland, Australia (on North Stradbroke Island) provides a case study in the (disastrous) environmental consequences on a fragile sandy-soil based ecosystem, justified by the provision of low wage casual labor on an island with few other work options.[
Sand mining contributes to the construction of buildings and development. However, the negative effects of sand mining include the permanent loss of sand in areas, as well as major habitat destruction.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 3:54 PM 0 comments
Oil shale

Oil shale, an organic-rich fine-grained sedimentary rock, contains significant amounts of kerogen (a solid mixture of organic chemical compounds) from which technology can extract liquid hydrocarbons. The name oil shale represents a double misnomer, as geologists would not necessarily classify the rock as a shale, and its kerogen differs from crude oil. Kerogen requires more processing to use than crude oil, which increases its cost as a crude-oil substitute both financially and in terms of its environmental impact. Deposits of oil shale occur around the world, including major deposits in the United States of America. Estimates of global deposits range from 2.8 trillion to 3.3 trillion barrels (450 × 109 to 520 × 109 m3) of recoverable oil.
The chemical process of pyrolysis can convert the kerogen in oil shale into synthetic crude oil. Heating oil shale to a sufficiently high temperature will drive off a vapor which processing can distill (retort) to yield a petroleum-like shale oil—a form of unconventional oil—and combustible oil-shale gas (the term shale gas can also refer to gas occurring naturally in shales). Industry can also burn oil shale directly as a low-grade fuel for power generation and heating purposes and can use it as a raw material in chemical and construction-materials processing.
Oil shale has gained attention as an energy resource as the price of conventional sources of petroleum has risen and as a way for some areas to secure independence from external suppliers of energy. At the same time, oil-shale mining and processing involve a number of environmental issues, such as land use, waste disposal, water use, waste-water management, greenhouse-gas emissions and air pollution. Estonia and China have well-established oil shale industries, and Brazil, Germany, Israel and Russia also utilize oil shale.
Geology
Oil shale, an organic-rich sedimentary rock, belongs to the group of sapropel fuels. It does not have a definite geological definition nor a specific chemical formula, and its seams do not always have discrete boundaries. Oil shales vary considerably in their mineral content, chemical composition, age, type of kerogen, and depositional history. Oil shale differs from bitumen-impregnated rocks (oil sands and petroleum reservoir rocks), humic coals and carbonaceous shale. While oil sands originate from the biodegradation of oil, heat and pressure have not (yet) transformed the kerogen in oil shale into petroleum.
Oil shale contains a lower percentage of organic matter than coal. In commercial grades of oil shale the ratio of organic matter to mineral matter lies approximately between 0.75:5 and 1.5:5. At the same time, the organic matter in oil shale has an atomic ratio of hydrogen to carbon (H/C) approximately 1.2 to 1.8 times lower than for crude oil and about 1.5 to 3 times higher than for coals. The organic components of oil shale derive from a variety of organisms, such as the remains of algae, spores, pollen, plant cuticles and corky fragments of herbaceous and woody plants, and cellular debris from other aquatic and land plants. Some deposits contain significant fossils; Germany's Messel Pit has the status of a Unesco World Heritage Site. The mineral matter in oil shale includes various fine-grained silicates and carbonates.
Geologists can classify oil shales on the basis of their composition as carbonate-rich shales, siliceous shales, or cannel shales. Another classification, known as the van Krevelen diagram, assigns kerogen types, depending on the hydrogen, carbon, and oxygen content of oil shales' original organic matter. The most commonly used classification of oil shales, developed between 1987 and 1991 by Adrian C. Hutton of the University of Wollongong, adapts petrographic terms from coal terminology. This classification designates oil shales as terrestrial, lacustrine (lake-bottom-deposited), or marine (ocean bottom-deposited), based on the environment of the initial biomass deposit. Hutton's classification scheme has proven useful in estimating the yield and composition of the extracted oil.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 3:51 PM 0 comments
Tuesday, July 28, 2009
Precious metal

A precious metal is a rare metallic chemical element of high economic value, which is classically not radioactive (to exclude natural Polonium, Radium, Actinium and Protactinium). Chemically, the precious metals are less reactive than most elements, have high lustre, are softer or more ductile, and have higher melting points than other metals. Historically, precious metals were important as currency, but are now regarded mainly as investment and industrial commodities. Gold, silver, platinum, and palladium each have an ISO 4217 currency code.
The best-known precious metals are gold and silver. While both have industrial uses, they are better known for their uses in art, jewellery and coinage. Other precious metals include the platinum group metals: ruthenium, rhodium, palladium, osmium, iridium, and platinum, of which platinum is the most widely traded.
The demand for precious metals is driven not only by their practical use, but also by their role as investments and a store of value. Historically, precious metals have commanded much higher prices than common industrial metals. In January 2009, gold was about $840/troy ounce and silver was about $11/troy ounce, compared to copper at $1.50/pound and nickel at $5/pound.
In the early part of the 21st century, precious metal prices rose significantly and recycling precious metals became more and more attractive. Although some companies have been doing recycling for many years, such as Sabin Metal Corporation since 1945.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 5:02 AM 0 comments
Phosphate

A phosphate, an inorganic chemical, is a salt of phosphoric acid. Inorganic phosphates are mined to obtain phosphorus for use in agriculture and industry. In organic chemistry, a phosphate, or organophosphate, is an ester of phosphoric acid. Organic phosphates are important in biochemistry.
Chemical properties
The phosphate ion is a polyatomic ion with the empirical formula PO43− and a molar mass of 94.973 g/mol. It consists of one central phosphorus atom surrounded by four identical oxygen atoms in a tetrahedral arrangement. The phosphate ion carries a negative three formal charge and is the conjugate base of the hydrogen phosphate ion, HPO42−, which is the conjugate base of H2PO4−, the dihydrogen phosphate ion, which in turn is the conjugate base of H3PO4, phosphoric acid. It is a hypervalent molecule (the phosphorus atom has 10 electrons in its valence shell). Phosphate is also an organophosphorus compound with the formula OP(OR)3. A phosphate salt forms when a positively-charged ion attaches to the negatively-charged oxygen atoms of the ion, forming an ionic compound. Many phosphates are not soluble in water at standard temperature and pressure. The sodium, potassium, rubidium, caesium and ammonium phosphates are all water soluble. Most other phosphates are only slightly soluble or are insoluble in water. As a rule, the hydrogenphosphates and the dihydrogenphosphates are slightly more soluble than the corresponding phosphates. The pyrophosphates are mostly water soluble.
In dilute aqueous solution, phosphate exists in four forms. In strongly-basic conditions, the phosphate ion (PO43−) predominates, whereas in weakly-basic conditions, the hydrogen phosphate ion (HPO42−) is prevalent. In weakly-acid conditions, the dihydrogen phosphate ion (H2PO4−) is most common. In strongly-acid conditions, aqueous phosphoric acid (H3PO4) is the main form.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 5:00 AM 0 comments
Saturday, July 25, 2009
Nickel

Nickel (pronounced /ˈnɪkəl/) is a chemical element, with the chemical symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. It is one of the four ferromagnetic elements at about room temperature. Its use has been traced as far back as 3500 BC, but it was first isolated and classified as a chemical element in 1751 by Axel Fredrik Cronstedt, who initially mistook its ore for a copper mineral. Its most important ore minerals are laterites, including limonite and garnierite, and pentlandite. Major production sites include Sudbury region in Canada, New Caledonia and Russia. The metal is corrosion-resistant, finding many uses in alloys, as a plating, in the manufacture of coins, magnets and common household utensils, as a catalyst for hydrogenation, and in a variety of other applications. Enzymes of certain life-forms contain nickel as an active center making the metal essential for them.
Characteristics
Nickel is a silvery-white metal with a slight golden tinge that takes a high polish. It is one of only four elements that are magnetic at or near room temperature. It belongs to the transition metals and is hard and ductile. It occurs most often in combination with sulfur and iron in pentlandite, with sulfur in millerite, with arsenic in the mineral nickeline, and with arsenic and sulfur in nickel galena. Nickel is commonly found in iron meteorites as the alloys kamacite and taenite. Similar to the elements chromium, aluminium and titanium, nickel is a very reactive element, but is slow to react in air at normal temperatures and pressures due to the formation of a protective oxide surface. Due to its permanence in air and its slow rate of oxidation, it is used in coins, for plating metals such as iron and brass, for chemical apparatus, and in certain alloys such as German silver.
Nickel is chiefly valuable for the alloys it forms, especially many superalloys, and particularly stainless steel. Nickel is also a naturally magnetostrictive material, meaning that in the presence of a magnetic field, the material undergoes a small change in length. In the case of nickel, this change in length is negative (contraction of the material), which is known as negative magnetostriction and is on the order of 50 ppm.
The most common oxidation state of nickel is +2 with several Ni complexes known. It is also thought that a +6 oxidation state may exist, however, this has not been demonstrated conclusively. The unit cell of nickel is a face centered cube with a lattice parameter of 0.352 nm giving a radius of the atom of 0.125 nm.
History
Because the ores of nickel are easily mistaken for ores of silver, understanding of this metal and its use dates to relatively recent times. However, the unintentional use of nickel is ancient, and can be traced back as far as 3500 BC. Bronzes from what is now Syria had contained up to 2% nickel. Further, there are Chinese manuscripts suggesting that "white copper" (cupronickel, known as baitung) was used there between 1700 and 1400 BC. This Paktong white copper was exported to Britain as early as the 17th century, but the nickel content of this alloy was not discovered until 1822.
In medieval Germany, a red mineral was found in the Erzgebirge (Ore Mountains) which resembled copper ore. However, when miners were unable to extract any copper from it they blamed a mischievous sprite of German mythology, Nickel (similar to Old Nick) for besetting the copper. They called this ore Kupfernickel from the German Kupfer for copper. This ore is now known to be nickeline or niccolite, a nickel arsenide. In 1751, Baron Axel Fredrik Cronstedt was attempting to extract copper from kupfernickel and obtained instead a white metal that he named after the spirit which had given its name to the mineral, nickel. In modern German, Kupfernickel or Kupfer-Nickel designates the alloy cupronickel.
In the United States, the term "nickel" or "nick" was originally applied to the copper-nickel Indian cent coin introduced in 1859. Later, the name designated the three-cent coin introduced in 1865, and the following year the five-cent shield nickel appropriated the designation, which has remained ever since. Coins of pure nickel were first used in 1881 in Switzerland.
After its discovery the only source for nickel was the rare Kupfernickel, but from 1824 on the nickel was obtained as byproduct of cobalt blue production. The first large scale producer of nickel was Norway, which exploited nickel rich pyrrhotite from 1848 on. The introduction of nickel in steel production in 1889 increased the demand for nickel and the nickel deposits of New Caledonia, which were discovered in 1865, provided most of the world's supply between 1875 and 1915. The discovery of the large deposits in the Sudbury Basin, Canada in 1883, in Norilsk-Talnakh , Russia in 1920 and in the Merensky Reef, South Africa in 1924 made large-scale production of nickel possible.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 9:44 AM 0 comments
Molybdenum

Molybdenum (pronounced /məˈlɪbdənəm/, from the Greek word for the metal "lead"), is a Group 6 chemical element with the symbol Mo and atomic number 42. The free element, which is a silvery metal, has the sixth-highest melting point of any element. It readily forms hard, stable carbides, and for this reason it is often used in high-strength steel alloys. Molybdenum does not occur as the free metal in nature, but rather in a variety of oxidation states in minerals. Industrially molybdenum compounds are used in high-pressure and temperature resistant greases between metals, as pigments, and catalysts.
Molybdenum minerals have long been known, but the element was "discovered" (in the sense of differentiating it as a new entity from minerals salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm.
Most of molybdenum's compounds have poor water-solubility, but the molybdate ion MoO2−4 is soluble, and will form if molybdenum-containing minerals are in contact with free oxygen and water. Recent theories suggest that the release of free oxygen by early life was important in removing molybdenum from minerals into a soluble form in the early oceans, where it was available to be used as a catalyst by single-celled organisms. This sequence may have been important in the history of life, because molybdenum-containing enzymes then became the most important catalysts used by some bacteria to break the bond in atmospheric molecular nitrogen, allowing biological nitrogen fixation. This, in turn allowed biologically driven nitrogen-fertilization of the oceans, and thus the development of more complex organisms. Aside from bacterial enzymes involved with nitrogen fixation, about 20 different molybdenum-containing enzymes are known today in animals. Molybdenum is a required element for life in these higher organisms, though not in all bacteria.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 9:43 AM 0 comments
Sunday, July 19, 2009
Marble

Marble is a nonfoliated metamorphic rock resulting from the metamorphism of limestone, composed mostly of calcite (a crystalline form of calcium carbonate, CaCO3). It is extensively used for sculpture, as a building material, and in many other applications. The word "marble" is colloquially used to refer to many other stones that are capable of taking a high polish.
Etymology
Venus de Milo, front.
The word "marble" derives from the Ancient Greek μάρμαρον (mármaron) or μάρμαρος (mármaros), "crystalline rock", "shining stone", from the verb μαρμαίρω (marmaírō), "to flash, sparkle, gleam". This stem is also the basis for the English word marmoreal, meaning "marble-like."
Origins
Marble is a metamorphic rock resulting from regional or rarely contact metamorphism of sedimentary carbonate rocks, either limestone or dolomite rock, or metamorphism of older marble. This metamorphic process causes a complete recrystallization of the original rock into an interlocking mosaic of calcite, aragonite and/or dolomite crystals. The temperatures and pressures necessary to form marble usually destroy any fossils and sedimentary textures present in the original rock.
Pure white marble is the result of metamorphism of very pure limestones. The characteristic swirls and veins of many colored marble varieties are usually due to various mineral impurities such as clay, silt, sand, iron oxides, or chert which were originally present as grains or layers in the limestone. Green coloration is often due to serpentine resulting from originally high magnesium limestone or dolostone with silica impurities. These various impurities have been mobilized and recrystallized by the intense pressure and heat of the metamorphism.
Construction marble
n the construction, specifically the dimension stone trade, the term "marble" is used for any crystalline calcitic rock (and some non-calcitic rocks) useful as building stone. For example, "Tennessee marble" is really a dense granular fossiliferous gray to pink to maroon Ordovician limestone that geologists call the Holston Formation.
Industrial use
Colorless or light-colored marbles are a very pure source of calcium carbonate, which is used in a wide variety of industries. Finely ground marble or calcium carbonate powder is a component in paper, and in consumer products such as toothpaste, plastics, and paints. Ground calcium carbonate can be made from limestone, chalk, and marble; about three-quarters of the ground calcium carbonate worldwide is made from marble. Ground calcium carbonate is used as a coating pigment for paper because of its high brightness and as a paper filler because it strengthens the sheet and imparts high brightness. Ground calcium carbonate is used in consumer products such as a food additive, in toothpaste, and as an inert filler in pills. It is used in plastics because it imparts stiffness, impact strength, dimensional stability, and thermal conductivity. It is used in paints because it is a good filler and extender, has high brightness, and is weather resistant. However, the growth in demand for ground calcium carbonate in the last decade has mostly been for a coating pigment in paper.
Calcium carbonate can also be reduced under high heat to calcium oxide (also known as "lime"), which has many applications including being a primary component of many forms of cement.
Production
According to the United States Geological Survey, U.S. dimension marble production in 2006 was 46,400 tons valued at $18.1 million, compared to 72,300 tons valued at $18.9 million in 2005. Crushed marble production (for aggregate and industrial uses) in 2006 was 11.8 million tons valued at $116 million, of which 6.5 million tons was finely ground calcium carbonate and the rest was construction aggregate. For comparison, 2005 crushed marble production was 7.76 million tons valued at $58.7 million, of which 4.8 million tons was finely ground calcium carbonate and the rest was construction aggregate. U.S. dimension marble demand is about 1.3 million tons. The DSAN World Demand for (finished) Marble Index has shown a growth of 12% annually for the 2000-2006 period, compared to 10.5% annually for the 2000–2005 period. The largest dimension marble application is tile.
Artificial marble
Faux marble or faux marbling is a wall painting technique that imitates the color patterns of real marble (not to be confused with paper marbling). Marble dust can be combined with cement or synthetic resins to make reconstituted or cultured marble.
Cultural associations
As the favorite medium for Greek and Roman sculptors and architects (see classical sculpture), marble has become a cultural symbol of tradition and refined taste. Its extremely varied and colorful patterns make it a favorite decorative material, and it is often imitated in background patterns for computer displays, etc.
Places named after the stone include Marblehead, Ohio; Marble Arch, London; the Sea of Marmara; India's Marble Rocks; and the towns of Marble, Minnesota; Marble, Colorado; and Marble Hill, Manhattan, New York. The Elgin Marbles are marble sculptures from the Parthenon that are on display in the British Museum. They were brought to Britain by the Earl of Elgin.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 2:35 AM 0 comments
Magnesite
Magnesite is magnesium carbonate, MgCO3. Iron (as Fe2+) substitutes for magnesium (Mg) with a complete solution series with siderite, FeCO3. Calcium, manganese, cobalt, and nickel may also occur in small amounts. Dolomite, (Mg,Ca)CO3, is almost indistinguishable from magnesite.
Occurrence
Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terranes. These magnesites often are cryptocrystalline and contain silica as opal or chert.
Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide within groundwaters.
Formation
Magnesite can be formed via talc carbonate metasomatism of peridotite and other ultrabasic rocks. Magnesite is formed via carbonation of olivine in the presence of water and carbon dioxide, and is favored at moderate temperatures and pressures typical of greenschist facies;
Magnesite can also be formed via the carbonation of magnesian serpentine (lizardite) via the following reaction:
Serpentine + carbon dioxide → Talc + magnesite + Water
2Mg3Si2O5(OH)4 + 3CO2 → Mg3Si4O10(OH)2 + 3MgCO3 + H2O
Forsterite magnesia-rich olivine compositions favor production of magnesite from peridotite. Fayalitic (iron-rich) olivine favors production of magnetite-magnesite-silica compositions.
Magnesite can also be formed from metasomatism in skarn deposits, in dolomitic limestones, associated with wollastonite, periclase, and talc.
Uses
Magnesite can be used as a slag former in steelmaking furnaces, in conjunction with lime, in order to protect the magnesium oxide lining. It can also be used as a catalyst and filler in the production of synthetic rubber and in the preparation of magnesium chemicals and fertilizers.
Similar to the production of lime, magnesite can be burned in the presence of charcoal to produce MgO, otherwise known as periclase. Such periclase is an important product in refractory materials.
Magnesite can also be used as a binder in flooring material.
In fire assay, Magnesite cupels can be used for cupellation as the Magnesite cupel will resist the high temperatures involved.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 2:33 AM 0 comments
Monday, July 13, 2009
Limestone

Limestone is a sedimentary rock composed largely of the mineral calcite (calcium carbonate: CaCO3). The deposition of limestone strata is often a by-product and indicator of biological activity in the geologic record. Calcium (along with nitrogen, phosphorus, and potassium) is a key mineral to plant nutrition: soils overlying limestone bedrock tend to be pre-fertilized with calcium. Limestone is an important stone for masonry and architecture, vying with only granite and sandstone to be the most commonly used architectural stone. Limestone is a key ingredient of quicklime, mortar, cement, and concrete. The solubility of limestone in water and weak acid solutions leads to important phenomena. Regions overlying limestone bedrock tend to have fewer visible groundwater sources (ponds and streams), as surface water easily drains downward through cracks in the limestone. While draining, water slowly (over thousands or millions of years) enlarges these cracks; dissolving the calcium-carbonate and carrying it away in solution. Most well-known natural cave systems are through limestone bedrock.
Description
Limestone often contains variable amounts of silica in the form of chert and/or flint, as well as varying amounts of clay, silt and sand as disseminations, nodules, or layers within the rock. The primary source of the calcite in limestone is most commonly marine organisms. These organisms secrete shells that settle out of the water column and are deposited on ocean floors as pelagic ooze or alternatively are conglomerated in a coral reef (see lysocline for information on calcite dissolution). Secondary calcite may also be deposited by supersaturated meteoric waters (groundwater that precipitates the material in caves). This produces speleothems such as stalagmites and stalactites. Another form taken by calcite is that of oolites (oolitic limestone) which can be recognized by its granular appearance.
Limestone makes up about 10% of the total volume of all sedimentary rocks. Limestones may also form in both lacustrine and evaporite depositional environments.
Calcite can be either dissolved by groundwater or precipitated by groundwater, depending on several factors including the water temperature, pH, and dissolved ion concentrations. Calcite exhibits an unusual characteristic called retrograde solubility in which it becomes less soluble in water as the temperature increases.
When conditions are right for precipitation, calcite forms mineral coatings that cement the existing rock grains together or it can fill fractures.
Karst topography and caves develop in carbonate rocks due to their solubility in dilute acidic groundwater. Cooling groundwater or mixing of different groundwaters will also create conditions suitable for cave formation.
Coastal limestones are often eroded by organisms which bore into the rock by various means. This process is known as bioerosion. It is most common in the tropics, and it is known throughout the fossil record (see Taylor and Wilson, 2003).
Because of impurities, such as clay, sand, organic remains, iron oxide and other materials, many limestones exhibit different colors, especially on weathered surfaces. Limestone may be crystalline, clastic, granular, or massive, depending on the method of formation. Crystals of calcite, quartz, dolomite or barite may line small cavities in the rock. Folk and Dunham classifications are used to describe limestones more precisely.
Travertine is a banded, compact variety of limestone formed along streams, particularly where there are waterfalls and around hot or cold springs. Calcium carbonate is deposited where evaporation of the water leaves a solution that is supersaturated with chemical constituents of calcite. Tufa, a porous or cellular variety of travertine, is found near waterfalls. Coquina is a poorly consolidated limestone composed of pieces of coral or shells.
During regional metamorphism that occurs during the mountain building process (orogeny) limestone recrystallizes into marble.
Limestone is a parent material of Mollisol soil group.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 8:51 PM 0 comments
Lead

Lead (pronounced /ˈlɛd/) is a main-group element with symbol Pb (Latin: plumbum) and atomic number 82. Lead is a soft, malleable poor metal, also considered to be one of the heavy metals. Lead has a bluish-white color when freshly cut, but tarnishes to a dull grayish color when exposed to air. It has a shiny chrome-silver luster when melted into a liquid.
Lead is used in building construction, lead-acid batteries, bullets and shot, weights, and is part of solder, pewter, fusible alloys and radiation shields. Lead has the highest atomic number of all stable elements, although the next element, bismuth, has a half-life so long (longer than the estimated age of the universe) it can be considered stable. Like mercury, another heavy metal, lead is a potent neurotoxin that accumulates in soft tissues and bone over time. Lead poisoning was documented in ancient Rome, Greece, and China.
Characteristics
Lead has a dull luster and is a dense, ductile, very soft, highly malleable, bluish-white metal that has poor electrical conductivity. This true metal is highly resistant to corrosion, and because of this property, it is used to contain corrosive liquids (e.g., sulfuric acid). Because lead is very malleable and resistant to corrosion it is extensively used in building construction, e.g., external coverings of roofing joints. Lead can be toughened by adding a small amount of antimony or other metals to it. It is a common misconception that lead has a zero Thomson effect. All lead, except 204Pb, is the end product of a complex radioactive decay. Lead is also poisonous, as are its compounds, and therefore is dangerous to human health and use as a food containment device is not recommended.
History
Lead has been commonly used for thousands of years because it is widespread, easy to extract and easy to work with. It is highly malleable and ductile as well as easy to smelt. Metallic lead beads dating back to 6400 B.C. have been found in Çatalhöyük in modern-day Turkey. In the early Bronze Age, lead was used with antimony and arsenic. Lead is mentioned in the Book of Exodus (15:10).
In alchemy, lead was thought to be the oldest metal and was associated with the planet Saturn. Lead pipes that bear the insignia of Roman emperors are still in service and many Roman "pigs" (ingots) of lead figure in Derbyshire lead mining history and in the history of the industry in other English centres. The Romans also used lead in molten form to secure iron pins that held together large limestone blocks in certain monumental buildings. Lead's symbol Pb is an abbreviation of its Latin name plumbum for soft metals; originally it was plumbum nigrum (literally, "black plumbum"), where plumbum candidum (literally, "bright plumbum") was tin. The English words "plumbing", "plumber", "plumb", and "plumb-bob" also derive from this Latin root.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 8:49 PM 0 comments
Saturday, July 11, 2009
Iron

Iron (pronounced /ˈаɪ.ərn/) is a chemical element with the symbol Fe (Latin: ferrum) and atomic number 26. Iron is a group 8 and period 4 element. Iron and iron alloys (steels) are by far the most common metals and the most common ferromagnetic materials in everyday use. Fresh iron surfaces are lustrous and silvery-grey in color, but oxidise in air to form a red or brown coating of ferrous oxide or rust. Compared with steel which has a hardness of about 140 Brinell, pure iron is soft, about 80 Brinell.
Iron-56 is the second heaviest stable isotope produced by the alpha process in stellar nucleosynthesis, the heaviest being nickel-62; heavier elements require a supernova for their formation. Iron is the most abundant element in the core of red giants, and is the most abundant metal in iron meteorites and in the dense metal cores of planets such as Earth.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 7:56 AM 0 comments
Diamond

In mineralogy, diamond (from the ancient Greek adámas, meaning "proper" or "unalterable") is an allotrope of carbon, where the carbon atoms are arranged in a variation of the face centered cubic crystal structure called a diamond lattice. Diamond is the second most stable form of carbon, after graphite; however, the conversion rate from diamond to graphite is negligible at ambient conditions. Diamond is specifically renowned as a material with superlative physical qualities, most of which originate from the strong covalent bonding between its atoms. In particular, diamond has the highest hardness and thermal conductivity of any bulk material synthesized so far. Those properties determine the major industrial application of diamond in cutting and polishing tools.
Diamond has remarkable optical characteristics. Because of its extremely rigid lattice, it can be contaminated by only few types of impurities, such as boron and nitrogen. Combined with the wide transparency (corresponding to the wide band gap of 5.5 eV), this results in clear, colorless appearance of most natural diamonds. Small amounts of defects or impurities (about one part per million) color diamond blue (boron), yellow (nitrogen), brown (lattice defects), green, purple, pink, orange or red. Diamond also has relatively high optical dispersion, that is ability to disperse light of different colors, which results in its characteristic luster. Excellent optical and mechanical properties, combined with efficient marketing, make diamond the most popular gemstone.
Most natural diamonds are formed at high-pressure high-temperature conditions existing at depths of 140 km to 190 km in the Earth mantle. Carbon-containing minerals provide the carbon source, and the growth occurs over periods from 1 billion to 3.3 billion years, which respectively corresponds to roughly 25% and 75% of the age of the Earth. Diamonds are brought close to the Earth surface through deep volcanic eruptions by a magma, which cools into igneous rocks known as kimberlites and lamproites. Diamonds can also be produced synthetically in a high-pressure high-temperature process which approximately simulates the conditions in the Earth mantle. An alternative, and completely different growth technique is chemical vapor deposition. Several non-diamond materials, which include cubic zirconia and silicon carbide and are often called diamond simulants, resemble diamond in appearance and many properties. Special gemological techniques have been specially developed to distinguish natural and synthetic diamonds and diamond simulants.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 7:55 AM 0 comments
Wednesday, July 8, 2009
Copper
Copper (pronounced /ˈkɒpər/) is a chemical element with the symbol Cu (Latin: cuprum) and atomic number 29. It is a ductile metal with very high thermal and electrical conductivity. Pure copper is rather soft and malleable and a freshly-exposed surface has a pinkish or peachy color. It is used as a thermal conductor, an electrical conductor, a building material, and a constituent of various metal alloys.
Copper metal and alloys have been used for thousands of years. In the Roman era, copper was principally mined on Cyprus, hence the origin of the name of the metal as Cyprium, "metal of Cyprus", later shortened to Cuprum. There may be insufficient reserves to sustain current high rates of copper consumption. Some countries, such as Chile and the United States, still have sizable reserves of the metal which are extracted through large open pit mines.
Copper compounds are known in several oxidation states, usually 2+, where they often impart blue or green colors to natural minerals such as turquoise and have been used historically widely as pigments. Copper as both metal and pigmented salt, has a significant presence in decorative art. Copper 2+ ions are soluble in water, where they function at low concentration as bacteriostatic substances and fungicides. For this reason, copper metal can be used as an anti-germ surface that can add to the anti-bacterial and antimicrobial features of buildings such as hospitals. In sufficient amounts, copper salts can be poisonous to higher organisms as well. However, despite universal toxicity at high concentrations, the 2+ copper ion at lower concentrations is an essential trace nutrient to all higher plant and animal life. In animals, including humans, it is found widely in tissues, with concentration in liver, muscle, and bone. It functions as a co-factor in various enzymes and in copper-based pigments.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 5:57 PM 0 comments
Coal
Coal is a readily combustible black or brownish-black sedimentary rock normally occurring in rock strata in layers or veins called coal beds. The harder forms, such as anthracite coal, can be regarded as metamorphic rock because of later exposure to elevated temperature and pressure. It is composed primarily of carbon along with variable quantities of other elements, chiefly sulfur, hydrogen, oxygen and nitrogen.
Coal was formed from layer upon layer of annual plant remains accumulating slowly that were protected from biodegradation by usually acidic covering waters that gave a natural antiseptic effect combating microorganisms and then later mud deposits protecting against oxidization in the widespread shallow seas — mainly during the Carboniferous period — thus trapping atmospheric carbon in the ground in immense peat bogs that eventually were covered over and deeply buried by sediments under which they metamorphosed into coal. In this manner, over time, the chemical and physical properties of the plant remains (believed to mainly have been fern-like species antedating more modern plant and tree species) were changed by geological action to create a solid material.
Coal, a fossil fuel, is the largest source of energy for the generation of electricity worldwide, as well as one of the largest worldwide anthropogenic sources of carbon dioxide emissions. Gross carbon dioxide emissions from coal usage are slightly more than those from petroleum and about double the amount from natural gas. Coal is extracted from the ground by mining, either underground or in open pits.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 5:56 PM 0 comments
Saturday, July 4, 2009
Chromite
Chromite is iron magnesium chromium oxide: (Fe, Mg)Cr2O4. It is an oxide mineral belonging to the spinel group. Magnesium can substitute for iron in variable amounts; also, aluminium and ferric iron commonly substitute for chromium.
Occurence
Chromite is found in peridotite from the Earth's mantle. It also occurs in layered ultramafic intrusive rocks. In addition, it is found in metamorphic rocks such as some serpentinites. Ore deposits of chromite form as early magmatic differentiates. It is commonly associated with olivine, magnetite, serpentine, and corundum. The vast Bushveld igneous complex of South Africa is a large layered mafic to ultramafic igneous body with some layers consisting of 90% chromite making the rare rock type, chromitite.
Uses
Chromite is also used as a refractory material, because it has a high heat stability.
The only ore of chromium is the mineral chromite. The two main products of Chromite refining are ferrochromium and metallic chromium, for those products the ore smelter process differs considerably. For the production of ferrochromium the chromite ore (FeCr2O4) is reduced with either aluminium or silicon in a aluminothermic reaction and for the production of pure chromium the iron has to be separated from the chromium in a two step roasting and leaching process.
Mining
In 2002 14,600,000 metric tons of chromite have been mined. The largest producers have been South Africa (44%) India (18%), Kazakhstan (16%) Zimbabwe (5%), Finland (4%) Iran (4%) and Brazil (2%) with several other countries producing the rest of less than 10% of the world production.
Minor production
In Pakistan, Chromite is mined from the ultramafic rocks in mainly the Muslim Bagh area of Zhob District of Balochistan. Most of the chromite is of metallurgical grade with Cr2O3 averaging 40% and a chrome to iron ratio of 2.6:1. Afghanistan has significant deposits of high grade Chromite ore.
Recently, the biggest user of Chromite ore has been China, importing large quantities from South Africa, Pakistan and other countries. The concentrate is used to make ferrochromium, which is in turn used to make Steel.
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 6:26 AM 0 comments
Bauxite

Bauxite is the most important aluminium ore. It consists largely of the minerals gibbsite Al(OH)3, boehmite γ-AlO(OH), and diaspore α-AlO(OH), together with the iron oxides goethite and hematite, the clay mineral kaolinite and small amounts of anatase TiO2. It was named after the village Les Baux in southern France, where it was first discovered in 1821 by the geologist Pierre Berthier.
Bauxite formation
Lateritic bauxites (silicate bauxites) are distinguished from karst bauxites (carbonate bauxites). The early discovered carbonate bauxites occur predominantly in Europe and Jamaica above carbonate rocks (limestone and dolomite), where they were formed by lateritic weathering and residual accumulation of intercalated clays or of clayey dissolution residues of the limestone.
The lateritic bauxites occur in many countries of the tropical belt. They were formed by lateritization (see laterite) of various silicate rocks such as granite, gneiss, basalt, syenite and shale. Compared with iron-rich laterites, the formation of bauxites demands even more intense weathering conditions with a very good drainage. This enables dissolution of kaolinite and precipitation of gibbsite. Zones with highest aluminium content are frequently located below a ferruginous surface layer. The aluminium hydroxide in the lateritic bauxite deposits is almost exclusively gibbsite.
Production trends
In 2007, Australia was one of the top producers of bauxite with almost one-third world share, followed by China, Brazil, Guinea, and Jamaica. Although aluminium demand is rapidly increasing, known reserves are sufficient to meet the needs for a considerable length of time. Increased aluminium recycling, which has the advantage of lowering the energy costs of production, will help extend bauxite reserves.
Processing
Bauxite is strip mined (surface mining) because it is found at the surface, with little or no overburden. Approximately 95% of the world's bauxite production is processed into aluminium. Bauxites are typically classified according to their intended commercial application: metallurgical, abrasive, cement, chemical and refractory.
Bauxites are heated in pressure vessels with sodium hydroxide solution at 150–200 °C through which aluminium is dissolved as aluminate (Bayer process). After separation of ferruginous residue (red mud) by filtering, pure gibbsite is precipitated when the liquor is cooled and seeded with fine grained aluminium hydroxide. Gibbsite is converted into aluminium oxide by heating. This is molten at approx. 1000 °C by addition of cryolite as a flux and reduced to metallic aluminium by a highly energy-consumptive electrolytic process (the Hall-Héroult process).
From http://en.wikipedia.org/
Labels: Materials mined
Posted by my blog at 6:23 AM 0 comments









